Quality PET Isotopes for Medical Diagnosis
Midwest Medical Isotopes is dedicated to shaping the future of nuclear medicine. Partner with us to harness innovation, regulatory excellence, and technical mastery for transformative advancements in healthcare.

Introduction
Midwest Medical Isotopes LLC stands as a leader in innovation and excellence within the field of nuclear medicine. As a prominent provider of cutting-edge radiopharmaceutical solutions, we offer a range of services that encompass state-of-the-art technology, stringent regulatory compliance, and a steadfast commitment to advancing healthcare through PET production and imaging. Located in Oklahoma City, centrally positioned in the USA, our facility serves a vast area, with a 1,000-mile radius covering a significant portion of the country, ensuring timely and efficient delivery of our radiopharmaceuticals.
Advanced Cyclotron Facility
Our world-class facility houses a state-of-the-art Siemens RDS 111 Eclipse medical cyclotron, enabling the production of a wide range of short-lived positron emission tomography (PET) isotopes. With precision engineering and advanced technology, we ensure the reliable and efficient generation of isotopes for the medical community
ISO Class 5 Certified HOT Cell
Our ISO Class 5 certified HOT cell is integral in transferring isotopes from the cyclotron to the appropriate radiotracer synthesizer module, ensuring precise dose preparation. This specialized environment guarantees the safe handling and manipulation of radiopharmaceuticals..
Radiotracer Synthesis Expertise
Midwest Medical Isotopes operates multiple radiotracer synthesizer modules, allowing us to efficiently synthesize a diverse range of PET radiopharmaceuticals. This capability accelerates the availability of critical radiotracers for disease diagnosis and treatment.
cGMP Quality Control Laboratory
Our cGMP-based quality control lab rigorously assesses the quality, stability, and safety of our radiopharmaceuticals. Equipped with an extensive suite of analytical tools, including analytical balances, endotoxin readers, osmometers, gas chromatographs, and HPLC, we perform comprehensive quality control assessments on our final products.
State-of-the-Art Clean Room Facility
Our cutting-edge clean room facility features an ante room and buffer room with an ISO Class 5 certified biosafety cabinet. This meticulous setup ensures contamination-free radiopharmaceutical production, safeguarding the integrity of our products.
Regulatory Excellence
As the only entity in the state with three FDA-approved ANDAs for PET radiopharmaceutical production (F-18 FDG, F-18 NaF, and N-13 NH3), we maintain exceptional regulatory compliance. We adhere to stringent FDA guidelines, DEQ standards, and local state board of pharmacy regulations.
Past Performance
Successfully delivered vital radiopharmaceuticals to major pharmacies and medical centers, enhancing diagnostic accuracy and patient care.
Achieved consistent compliance with rigorous regulatory standards, resulting in FDA approval for multiple ANDA’s.
Received certification by US National Cancer Institute for manufacturing trial of a new bone marrow and brain imaging agent (F-18 Fluorothymidine or FLT). This work had a great importance for the study of bone cancers and aggressive brain tumors.
Services We Offer:
- Contract Manufacturing Services
- Precursor and Intermediate Synthesis
- Kits Manufacturing and Assembly
- Research and Development Support
- Analytical Services

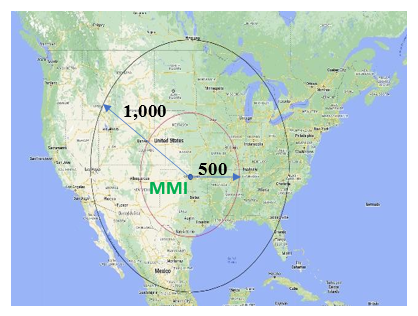
Our Location
- Located in the heart of the United States, our facility offers a strategic logistical advantage with access to a large portion of the country within a 1,000-mile radius. This central location enables efficient manufacturing of kits, precursors, intermediates, and final products, while streamlining shipping to reduce transit times and optimize nationwide distribution.


